2020-11-23 19:57:53
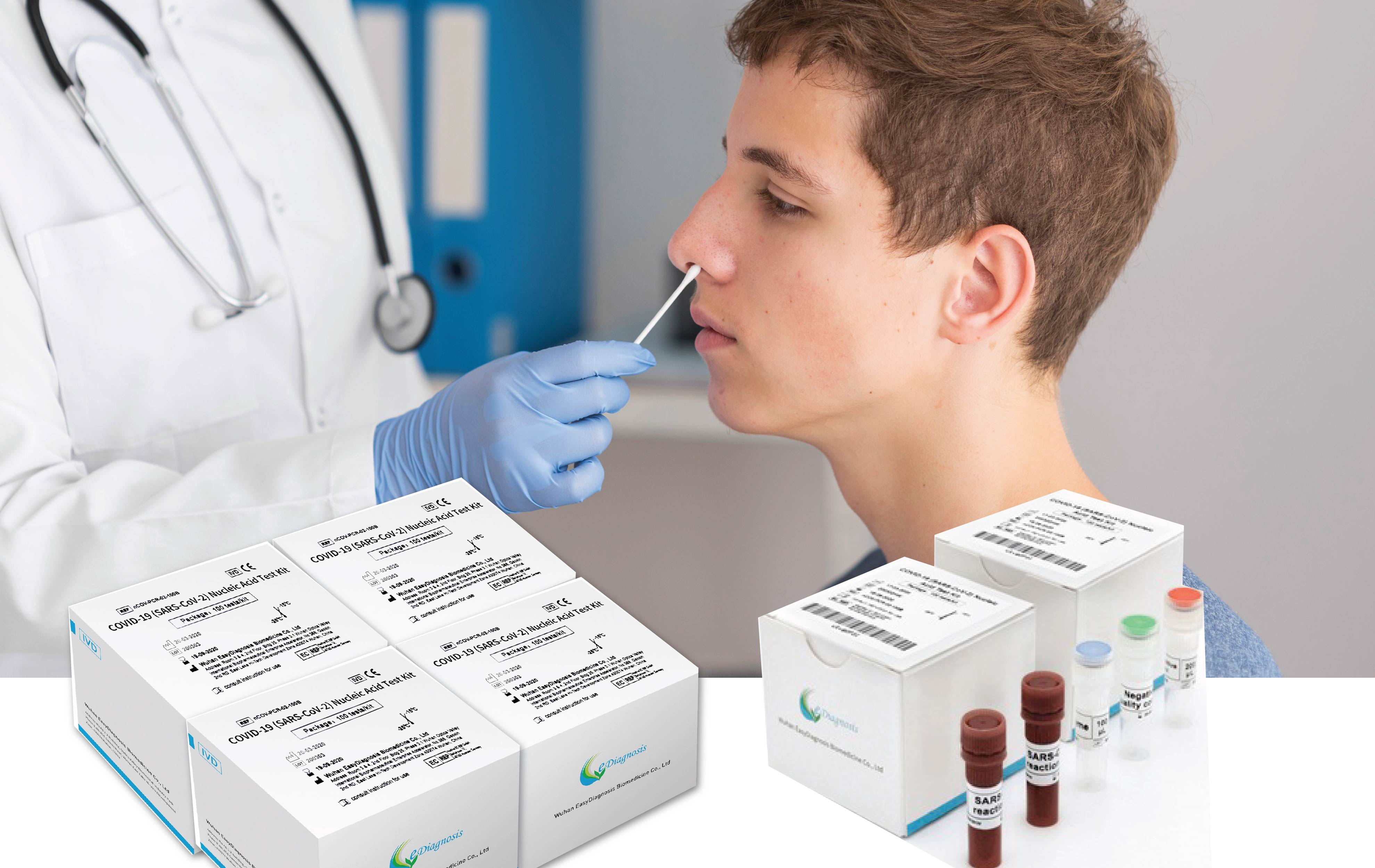
KUALA LUMPUR, 23 NOVEMBER 2020 – Ho Wah Genting Berhad (“HWGB” or the “Company”) (Bursa Stock Code:9601) is pleased to announce that the nucleic acid test kits manufactured by Wuhan Easydiagnosis Biomedicine Co Ltd (“Wuhan Easydiagnosis”) has been approved for distribution in Malaysia.
HWGB Biotech Sdn Bhd’s General Manager, Medical & Pharmaceutical Research, Dr. Yaman Walid Kassab, said: “The approval by the MOH of Wuhan Easydiagnosis’s nucleic acid test kits came at a crucial time as Malaysia has seen a rise in COVID-19 infections in recent months compared to earlier in the year. When these test kits become available, it will help to ease the backlog of samples that needed to be tested and therefore, make it faster for results to be known. We are glad that we had received the first sale and it is now officially in the market”.
Wuhan Easydiagnosis is backed by a well-established research and development team together with 100,000 production lines in international-grade facilities. It is also the first manufacturer of bedside, rapid and quantitative blood-detectors in China while more than 30 of its Point-of-Care Test (“POCT”) products are approved and registered with China’s State Food and Drug Administration.

Ho Wah Genting Berhad (“HWGB” or the “Company”) is principally engaged in investment holdings and the provision of management services to its subsidiaries. The Company had on 30 June 2020 diversified its existing businesses to include healthcare related industry which mainly involved in health supplement, biotechnology and health technology. In addition, the Company and its subsidiaries (“HWGB Group” or the “Group”) are also engaging in the businesses of investment holdings; manufacturing of wires and cables and moulded power supply cord sets and cable assemblies for electrical and electronic devices and equipment; trading of wires and cables; and travel agent and tour related services.

HWGB Biotech Sdn Bhd, was incorporated on 18 June 2012 under the Companies Act, 1965 under the former name of HWG Consortium Sdn Bhd and changed to its current name on 22 May 2020. HWGB Biotech is principally involved in the ddistribution of all kinds of biotechnology products, bioinformatics diagnostic tools, all medical engineering equipment along with software developments and tools as well as to patent all original products and by-products, technologies and software developed or sourced by the company.
Dr.Yaman Walid Kassab is an assistant Professor of Clinical Pharmacy. He obtained his PhD in Clinical Pharmacy at University Science Malaysia (USM) in 2013. He possesses 7 years of experience in the academia, specifically in the pharmacotherapy field. He is actively involved in medical research and publication and has published more than 40SCOPUS/ISI indexed research papers and has supervised more than 50 research projects and 20 postgraduate research students. He is also serving as an editorial Board member of several reputed international journals like “Frontiers in Pharmacology”, “Archives of Pharmacy Practice (APP)” , “Journal of Medical and Pharmaceutical Innovation (JMPI)” , ”Annals of Cardiology and Cardiovascular Diseases” , “Updates in Public Health and Preventive Medicine” He has been a member of many international professional bodies such as International Association of Therapeutic Drug Monitoring & Clinical Toxicology in CANADA, Syndicate of Syrian Pharmacists, Malaysian Association of Education in Medicine & Health Sciences, Malaysian Pharmaceutical Society.
Back